
Order the NovaSeq X Series
Advanced chemistry, optics, and informatics combine to deliver exceptional speed and data quality, outstanding throughput and scalability.
Enable CGP with a pan-cancer panel capable of detecting key IO gene signatures (TMB, MSI) plus all main variant classes from ctDNA in blood plasma.
Assay time
Hands-on time
Input quantity
This product is only available in select locations. Please select the location where you would like this product to be shipped to see availability.
TruSight Oncology 500 ctDNA v2 is now available with improved sensitivity using lower cfDNA input, a faster turnaround time, and a more streamlined workflow.
TruSight Oncology 500 ctDNA v2 is a pan-cancer NGS assay that enables in-house comprehensive genomic profiling (CGP) of circulating tumor DNA (ctDNA) in blood plasma for research.
Achieve sensitive and accurate detection (0.2% VAF for SNVs) with low inputs (20 ng) using UMI-based hybrid-capture library prep and deep sequencing.
DRAGEN secondary analysis powers a rapid variant calling algorithm on-premises or in the cloud with Illumina Connected Analytics. Access insights using integrated reporting with Illumina Connected Insights* or Velsera.
Advanced chemistry and extensive workflow optimization enable a single-day library prep plus enrichment for a total turnaround time of <4 days. Automation-friendly kits and methods enable increased efficiencies.
*Not available in all countries. Illumina Connected Insights supports user-defined tertiary analysis through API calls to third-party knowledge sources.
Maximize chances of identifying an actionable alteration with the TruSight Oncology 500 product line.
TruSight Oncology 500 ctDNA v2
Analyze circulating tumor DNA (ctDNA) in blood plasma with similar DNA panel content as TruSight Oncology 500.
Assess relevant DNA and RNA cancer biomarkers from FFPE tumor tissue, now including HRD through an accessory kit to assess the Genomic Instability Score (GIS).†
TruSight Oncology 500 High-Throughput Assay
Batch up to 192 samples at a time while using the same panel content and tissue input type as TruSight Oncology 500.
†HRD is only available with the addition of the TruSight Oncology 500 HRD kit to the TruSight Oncology 500 kit. Not available in Japan.
| Analytical sensitivity | ≥ 95% (small variants, ≥ 0.5% VAF) |
|---|---|
| Analytical specificity | ≥ 99.995% (small variants, ≥ 0.5% VAF) |
| Assay time | 3-4 days from purified nucleic acid to variant report |
| Cancer type | Pan-cancer, Solid tumor |
| Content specifications |
Targeted selection of 523 genes (full coding sequence) for a total of 1.94Mb panel size. • Immuno-oncology biomarker coverage: TMB and MSI • Guideline coverage: Broad coverage of key guidelines for multiple solid tumor types • Clinical trial coverage: Over 600 clinical trials (based on Pierian clinical knowledgebase, as of February 2023) |
| Description | Provides a noninvasive method for profiling solid tumors for cancer research applications through comprehensive genomic profiling of liquid biopsy samples (ctDNA from blood plasma). This liquid biopsy approach provides insights about intra- and inter-tumor heterogeneity using a minimally invasive sample collection approach to complement tissue-based CGP. |
| Gene count | 523 genes for DNA |
| Hands-on time | ~1.5-2.5 hr for library prep and enrichment |
| Input quantity | 20 ng cfDNA (4 ml of plasma) |
| Instruments | NovaSeq 6000 System |
| Mechanism of action | Hybrid-capture chemistry |
| Method | Targeted DNA sequencing, Target enrichment |
| Multiplexing | Up to 8 on S2, and 24 on S4 using Xp-4 Lane workflow, 16 indexes maximum |
| Nucleic acid type | DNA |
| Specialized sample types | Circulating tumor DNA, Blood |
| Species category | Human |
| Technology | Sequencing |
| Variant class | Single nucleotide variants (SNVs), Insertions-deletions (indels), Copy number variants (CNVs) |
To run TruSight Oncology 500 ctDNA v2, you need:
To analyze with the DRAGEN pipeline, you need:
To generate a final variant report, you need:
* Not available in all countries. Illumina Connected Insights supports user-defined tertiary analysis through API calls to third-party knowledge sources.
† Velsera was previously known as Pierian. Other commercial options are available.
TruSight Oncology 500 ctDNA v2 Assay
*Novaseq X and NovaSeq 6000Dx (in RUO mode) compatibility to come in 2024.
†Not available in all countries. Illumina Connected Insights supports user-defined tertiary analysis through API calls to third-party knowledge sources.
| Instrument | Recommended number of samples | Read length |
|---|---|---|
| NovaSeq 6000 System | 24 samples per run (S4 flow cell), 800M paired-end reads, 35,000x coverage |
2 × 150 bp |
| NovaSeq 6000 System | 8 samples per run (S2 flow cell), 800M paired-end reads, 35,000x coverage |
2 × 150 bp |
Next-generation sequencing provides deep insights into the molecular underpinnings of tumors and can help advance the promise of personalized medicine.
Pathology and clinical cancer research
Our clinical cancer research solutions deliver accurate genomic information, and enable labs to analyze multiple genes in a single test.
| TruSight Oncology 500 ctDNA v2 | TruSight Oncology 500 | TruSight Oncology 500 High-Throughput | |
|---|---|---|---|
| Analytical sensitivity | ≥ 95% (small variants, ≥ 0.5% VAF) | ||
| Analytical specificity | ≥ 99.995% (small variants, ≥ 0.5% VAF) | ||
| Assay time | 3-4 days from purified nucleic acid to variant report | 4–5 days from sample to results | 4–5 days from sample to results |
| Cancer type | Pan-cancer, Solid tumor | Pan-cancer, Solid tumor | Pan-cancer, Solid tumor |
| Content specifications |
Targeted selection of 523 genes (full coding sequence) for a total of 1.94Mb panel size. • Immuno-oncology biomarker coverage: TMB and MSI • Guideline coverage: Broad coverage of key guidelines for multiple solid tumor types • Clinical trial coverage: Over 600 clinical trials (based on Pierian clinical knowledgebase, as of February 2023) |
Targeted sequencing of DNA from 523 genes and RNA from 55 genes for a total of 1.94 Mb panel size. MSI and TMB measurement included. The optional TruSight Oncology 500 HRD kit (not available in Japan) content includes coverage of ~25K SNPs to assess homologous recombination deficiency through a comprehensive genomic instability score (LOH+TAI+LST) powered by Myriad Genetics. | Targeted sequencing of DNA from 523 genes of interest and RNA from 55 genes, for a total of 1.94Mb panel size. MSI and TMB measurement included. |
| Description | Provides a noninvasive method for profiling solid tumors for cancer research applications through comprehensive genomic profiling of liquid biopsy samples (ctDNA from blood plasma). This liquid biopsy approach provides insights about intra- and inter-tumor heterogeneity using a minimally invasive sample collection approach to complement tissue-based CGP. | Assay that enables comprehensive genomic profiling from FFPE tissue and runs on the NextSeq 550 System or NextSeq 550Dx Instrument (in Research Mode) and can batch up to eight samples at a time. | A high-throughput comprehensive NGS assay to identify key biomarkers in guidelines and > 1K clinical trials from a streamlined workflow using the NovaSeq 6000 System or NovaSeq 6000Dx Instrument (in Research Mode). Includes coverage of immuno-oncology biomarkers MSI and TMB. |
| Gene count | 523 genes for DNA | ||
| Hands-on time | ~1.5-2.5 hr for library prep and enrichment |
~2.5 hr for automated workflow ~10.5 hr for manual workflow |
~2.5 hr for automated workflow ~10.5 hr for manual workflow |
| Input quantity | 20 ng cfDNA (4 ml of plasma) | 40 ng DNA and/or 40 ng RNA | 40 ng DNA and/or 40–80 ng RNA |
| Instruments | NovaSeq 6000 System | NextSeq 550 System, NextSeq 550Dx in Research Mode, NextSeq 500 System | NovaSeq 6000Dx in Research Mode, NovaSeq 6000 System |
| Mechanism of action | Hybrid-capture chemistry | ||
| Method | Targeted DNA sequencing, Target enrichment | Targeted DNA sequencing, Targeted RNA sequencing, Target enrichment | Targeted DNA sequencing, Targeted RNA sequencing, Target enrichment |
| Multiplexing | Up to 8 on S2, and 24 on S4 using Xp-4 Lane workflow, 16 indexes maximum | Up to 8-plex | Up to 16-plex (SP flow cell), 32-plex (S1 flow cell), 72-plex (S2 flow cell), and 192-plex (S4 flow cell) |
| Nucleic acid type | DNA | DNA, RNA | DNA, RNA |
| Specialized sample types | Circulating tumor DNA, Blood | FFPE tissue | FFPE tissue |
| Species category | Human | Human | Human |
| Technology | Sequencing | Sequencing | Sequencing |
| Variant class | Single nucleotide variants (SNVs), Insertions-deletions (indels), Copy number variants (CNVs) | Gene fusions, Transcript variants, Single nucleotide variants (SNVs), Insertions-deletions (indels), Copy number variants (CNVs), Loss of heterozygosity (LOH), Somatic variants, Novel transcripts, Single nucleotide polymorphisms (SNPs), Structural variants | Gene fusions, Somatic variants, Novel transcripts, Structural variants, Transcript variants, Single nucleotide variants (SNVs), Insertions-deletions (indels), Copy number variants (CNVs) |
* Based on Pierian clinical knowledgebase, as of February 2023.
* NovaSeq 6000Dx System in RUO Mode requires a separate, stand-alone DRAGEN server for secondary analysis.
* NovaSeq X and NovaSeq 6000 Dx compatibility coming in 2024
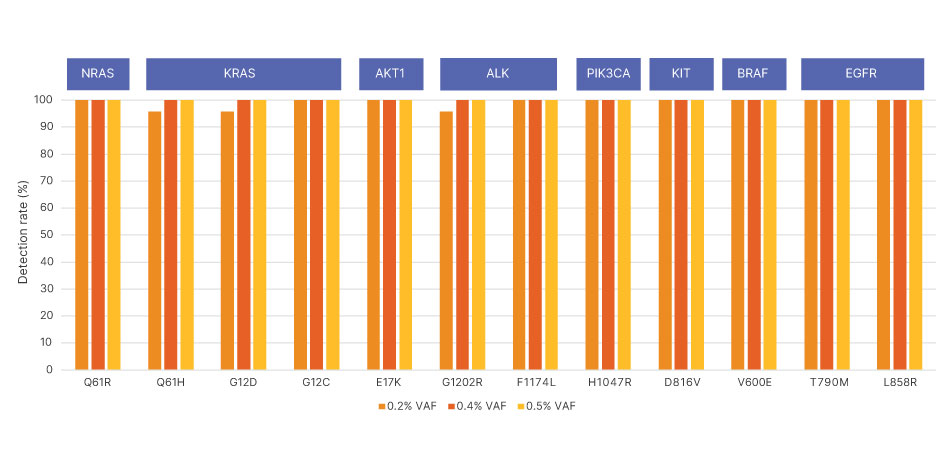

Synthetic control samples with known variant allele frequency (VAF) for each single nucleotide variant were diluted to values ranging from 0.20%–0.50% VAF and analyzed by TruSight Oncology 500 ctDNA v2.
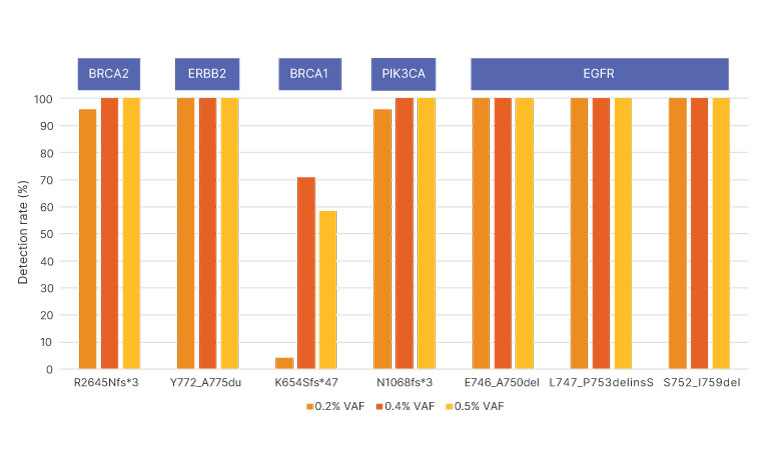

Synthetic control samples with known VAF for each insertion or deletion were diluted to values ranging from 0.20%–0.50% VAF and analyzed by TruSight Oncology 500 ctDNA v2.
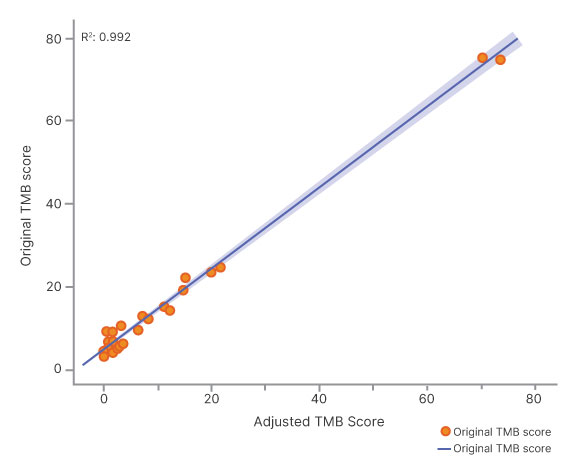

Tumor-only workflow of TSO 500 ctDNA v2 utilizing advanced bioinformatics for germline and CH variant filtering produces highly concordant bTMB compared to a tumor-normal workflow.
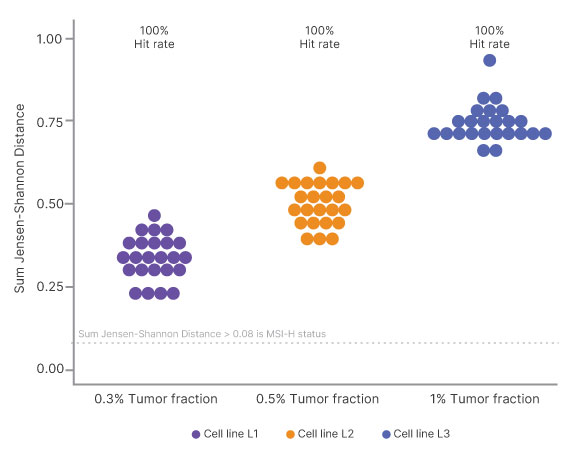

Microsatellite instability (MSI) was evaluated in 3 cell lines with known MSI-high status (samples 1–3) and detected down to 0.3% tumor fraction assessing up to ~2300 homopolymer sites.
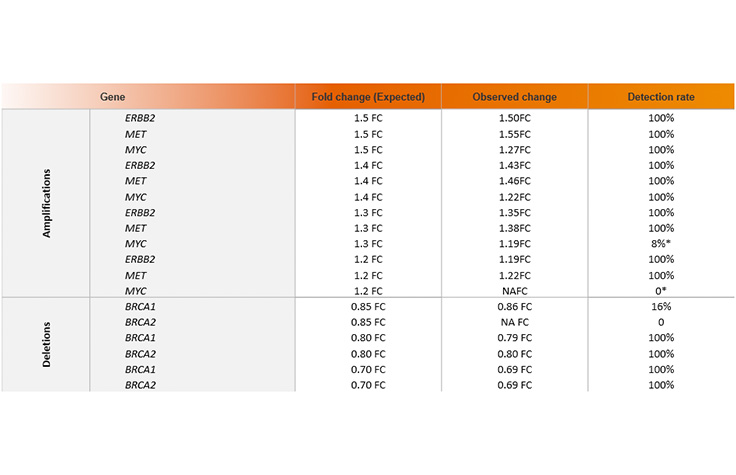

Lower relative MYC detection due to limit of detection approached faster due to fewer starting copies. Illumina data on file, 2023.
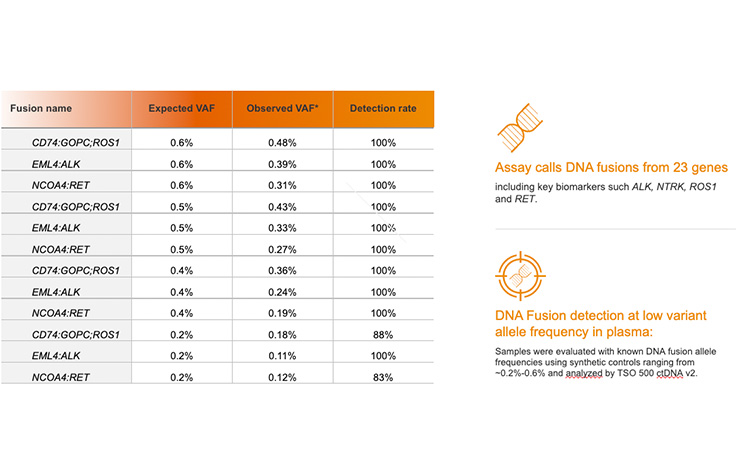

VAF calculated by dividing the total number of supporting reads by the higher depth of the two sides of the breakpoint. Illumina data on file, 2023.
Illumina improved the analysis of circulating tumors with TruSight Oncology ctDNA 500 v2. Learn about new features and benefits in the video.

Interested in learning more about the TruSight Oncology 500 portfolio of products?
Illumina collaborates with National Cancer Center Japan to address a leading cause of death in Asia
Learn about this joint effort to accelerate the development of personalized treatments based on genomic information.
TruSight Oncology 500 ctDNA v2 (24 samples)
20105899
TSO 500 ctDNA v2 Manual Kit
List Price:
Discounts:
TruSight Oncology 500 ctDNA v2 for Use with NovaSeq 6000 S2 Flow Cell (24 samples)
20105901
Mixed Workflow TSO 500 ctDNA v2 + S2 FC Manual Kit
List Price:
Discounts:
TruSight Oncology 500 ctDNA v2 for Use with NovaSeq 6000 S4 Flow Cell (24 samples)
20105902
Mixed Workflow TSO 500 ctDNA v2 + S4 FC Manual Kit
List Price:
Discounts:
TruSight Oncology 500 ctDNA v2 (24 samples) plus Velsera Interpretation Report
20105905
TSO 500 ctDNA v2 Manual Kit + Velsera Interpretation Report
List Price:
Discounts:
TruSight Oncology 500 ctDNA v2 plus Velsera Interpretation Report, for Use with NovaSeq 6000 S2 Flow Cell (24 samples)
20105907
Mixed Workflow TSO 500 ctDNA v2 + S2 FC Manual Kit + Velsera Interpretation Report
List Price:
Discounts:
TruSight Oncology 500 ctDNA v2 plus Velsera Interpretation Report, for Use with NovaSeq 6000 S4 Flow Cell (24 samples)
20105908
Mixed Workflow TSO 500 ctDNA v2 + S4 FC Manual Kit + Velsera Interpretation Report
List Price:
Discounts:
TruSight Oncology 500 ctDNA Kit (48 samples)
20039252
TruSight Oncology 500 ctDNA Kit (Includes DNA library prep and enrichment reagents. Does not include NovaSeq core reagents)
List Price:
Discounts:
TruSight Oncology 500 ctDNA Kit plus Velsera Interpretation Report (16 indexes, 48 Samples)
20043410
Includes library prep and enrichment reagents, plus data interpretation reports (through Velsera Clinical Genomics Workspace software). Does not include NovaSeq System Core Reagents.
List Price:
Discounts:
TruSight Oncology 500 ctDNA Kit, for use with NovaSeq 6000 S2 (48 samples)
20101995
Combination SKU for TSO500 ctDNA and the NovaSeq 6000 S2 Core consumables
List Price:
Discounts:
TruSight Oncology 500 ctDNA Kit, for use with NovaSeq 6000 S4 (48 samples)
20101998
Combination SKU for TSO500 ctDNA and the NovaSeq 6000 S4 Core consumables
List Price:
Discounts:
TruSight Oncology 500 ctDNA Kit for use with NovaSeq 6000 S2 plus Velsera interpretation report (16 indexes, 48 samples)
20102000
Combination SKU for TSO500 ctDNA and the NovaSeq 6000 S2 Core consumables + Velsera report
List Price:
Discounts:
TruSight Oncology 500 ctDNA Kit for use with NovaSeq 6000 S4 plus Velsera interpretation report (16 indexes, 48 samples)
20102001
Combination SKU for TSO500 ctDNA and the NovaSeq 6000 S4 Core consumables + Velsera report
List Price:
Discounts:
IDT® for Illumina® UMI DNA/RNA UD Indexes Set A, Ligation (96 Indexes, 96 Samples)
20034701
UMI DNA Index Anchors (Plate = 20027219, Box = 20032119) + Nextera Compatible Unique Dual Index A (Sales Kit = 20027213, Plate = 20025019, Box = 20026121)
List Price:
Discounts:
IDT® for Illumina® UMI DNA/RNA UD Indexes Set B, Ligation (96 Indexes, 96 Samples)
20034702
IDT for Illumina UMI DNA Index Anchors Set B
List Price:
Discounts:
IDT for Illumina UMI DNA/DNA Index Anchors Set A for Automation
20066404
Includes one box of 96 IDT for Illumina - UMI DNA/RNA UD Indexes Set A for Automation sufficient for labeling 96 samples and one box of 96 IDT for Illumina - UMI DNA Index Anchors for Automation.
List Price:
Discounts:
IDT for Illumina UMI DNA/DNA Index Anchors Set B for Automation
20063213
Includes one box of 96 IDT for Illumina - UMI DNA/RNA UD Indexes Set B for Automation sufficient for labeling 96 samples and one box of 96 IDT for Illumina - UMI DNA Index Anchors for Automation.
List Price:
Discounts:
NovaSeq 6000 S2 Reagent Kit v1.5 (300 cycles)
20028314
Includes one S2 flow cell, one buffer cartridge, one cluster cartridge, and one sequencing cartridge to support a 300-cycle run on the NovaSeq 6000 System.
List Price:
Discounts:
NovaSeq 6000 S4 Reagent Kit v1.5 (300 cycles)
20028312
Includes one S4 flow cell, one buffer cartridge, one cluster cartridge, and one sequencing cartridge to support a 300-cycle run on the NovaSeq 6000 System.
List Price:
Discounts:
Illumina DRAGEN Server v4
20051343
Includes Avance Exchange support for the first year. Requires purchase of annual DRAGEN license.
List Price:
Discounts:
ICA Basic Annual Subscription
20044874
Illumina Connected Analytics (ICA) Basic Annual Subscription. This product includes 1 year of access to ICA Basic, including sequencing instrument connectivity, data management capabilities, and access to pre-packaged analysis tools.
ICA Professional Annual Subscription
20044876
Illumina Connected Analytics (ICA) Professional Annual Subscription. This product includes 1 year of access to ICA, including sequencing instrument connectivity, data management capabilities, access to pre- packaged tools, and the ability to create customized workflows composed of tools, pipelines, data warehouses, and notebooks.
ICA Enterprise Annual Subscription
20038994
Illumina Connected Analytics (ICA) Enterprise Annual Subscription. This product includes 1 year of access to ICA Enterprise, including sequencing instrument connectivity, data management capabilities, custom and pre-packaged analysis tools, and the Base module for data warehousing and mining. ICA Enterprise also includes optional HIPAA BAA (US-only), single sign-on (SSO), and a service level agreement (SLA).
ICA Enterprise Srvc & Compliance Add-on
20066830
Illumina Connected Analytics (ICA) Compliance enables single sign-on (SSO), multi-factor authentication (MFA), HIPAA BAA (US-only), and a Service Level Agreement (SLA) for an ICA Basic Annual Subscription.
Illumina Analytics - 1 iCredit
20042038
iCredits are used for data storage and analysis on either BaseSpace Sequence Hub or Illumina Connected Analytics.
List Price:
Discounts:
Illumina Analytics Starter Pack - 1,000 iCredits
20042039
iCredits are used for data storage and analysis on either BaseSpace Sequence Hub or Illumina Connected Analytics.
List Price:
Discounts:
Illumina Analytics - 5,000 iCredits
20042040
iCredits are used for data storage and analysis on either BaseSpace Sequence Hub or Illumina Connected Analytics.
List Price:
Discounts:
Illumina Analytics - 50,000 iCredits
20042041
iCredits are used for data storage and analysis on either BaseSpace Sequence Hub or Illumina Connected Analytics.
List Price:
Discounts:
Illumina Analytics - 100,000 iCredits
20042042
iCredits are used for data storage and analysis on either BaseSpace Sequence Hub or Illumina Connected Analytics.
List Price:
Discounts:
Insights - Annual Subscription
20090137
Annual subscription to Illumina Connected Insights platform.
Illumina Connected Insights - Research – Trial Subscription
20112516
Free trial subscription for Illumina Connected Insights - Research for oncology.
Illumina Connected Insights – Oncology Genome Equivalent Sample - VCF
20090138
Illumina Connected Insights pre-paid oncology samples on per genome equivalent basis starting from VCF: one genome is equivalent to 2 exomes, 3 large and 6 small panel samples. Any unused samples will automatically roll over provided that the annual subscription to Illumina Connected Insights (20090137) is renewed on an annual basis. Access to a set of oncology knowledge bases is included.
Connected Insights Training - Remote
20092376
Illumina Connected Insights Training - Remote includes five (5) hours of product training delivered virtually.
Informatics Professional Services
20071787
Professional Services rendered for Illumina informatics products and solutions, defined by a statement of work.
TruSight Oncology 500 ctDNA v2 for Automation (48 samples)
20105900
TSO 500 ctDNA v2 AutomatedKit
List Price:
Discounts:
TruSight Oncology 500 ctDNA v2 Automation Kit, for Use with NovaSeq 6000 S2 Flow Cell (48 samples)
20105903
Mixed Workflow TSO 500 ctDNA v2 + S2 FC Automated Kit
List Price:
Discounts:
TruSight Oncology 500 ctDNA v2 Automation Kit, for Use with NovaSeq 6000 S4 Flow Cell (48 samples)
20105904
Mixed Workflow TSO 500 ctDNA v2 + S4 FC Automated Kit
List Price:
Discounts:
TruSight Oncology 500 ctDNA v2 for Automation (48 samples) plus Velsera Interpretation Report
20105906
TSO 500 ctDNA v2 AutomatedKit + Velsera Interpretation Report
List Price:
Discounts:
TruSight Oncology 500 ctDNA v2 Automation Kit plus Velsera Interpretation Report, for Use with NovaSeq 6000 S2 Flow Cell (48 samples)
20105909
Mixed Workflow TSO 500 ctDNA v2 + S2 FC Automated Kit + Velsera Interpretation Report
List Price:
Discounts:
TruSight Oncology 500 ctDNA v2 Automation Kit plus Velsera Interpretation Report, for Use with NovaSeq 6000 S4 Flow Cell (48 samples)
20105910
Mixed Workflow TSO 500 ctDNA v2 + S4 FC Automated Kit + Velsera Interpretation Report
List Price:
Discounts:
Informatics Professional Services
20071787
Professional Services rendered for Illumina informatics products and solutions, defined by a statement of work.
Connected Insights Training - Remote
20092376
Illumina Connected Insights Training - Remote includes five (5) hours of product training delivered virtually.
Showing of
Product
Qty
Unit Price
Product
Catalog ID
Quantity
Unit price
TruSight Oncology 500 ctDNA v2 is an ultra-sensitive, streamlined liquid biopsy assay for solid tumors that enables CGP from blood plasma samples in <4 days. TruSight Oncology 500 ctDNA v2 offers improved sensitivity using a lower cfDNA input, a faster turnaround time, and a more streamlined workflow compared to the original TruSight Oncology 500 ctDNA assay. TruSight Oncology 500 and TruSight Oncology 500 High-Throughput enable CGP from tissue samples; see other differences in the TruSight Oncology assay comparison table.
The TruSight Oncology 500 ctDNA v2 analysis workflow uses an off-instrument software run on the DRAGEN v4 or v3 server or Illumina Connected Analytics platform to generate sample outputs, including high-level sample metrics, variants detected, and TMB and MSI scores. Tertiary analysis is enabled with either Illumina Connected Insights or the Velsera CGW.
A BaseSpace Sequence Hub evaluation app is also available for assessment use only. Illumina has no obligation to provide technical support for this app. Access is limited to 30 days.
Learn more about compatible analysis software products.
Analysis on the DRAGEN server takes approximately 20-24 hours for S4 runs with 24 libraries and approximately 9–12 hours for S2 runs with 8 libraries.
Yes, you can perform analysis with your own software. However, Illumina will not be able to directly provide technical support in this case.
Approximately 20 ng input cfDNA is recommended to achieve ~0.2% VAF detection for SNVs at >90% sensitivity and >95% specificity; however, the assay can accept a range of input from 10-30 ng cfDNA.
Reach out for information about our products and services, or get answers to questions about our technology.
Your email address is never shared with third parties.